Lightning
There’s more going on than just a dangerous light show.
Yet another wild thunderstorm of the summer of 2023 had rolled over our location. My phone weather app noted that some of the lightning strikes hit just a few hundred yards from us and the rain gauge showed nearly 2 inches had fallen in less than an hour. As the storm moved south and east, we could see the massive thunderhead churning the ingredients of cold air, warm air, water vapor, ice, friction, and ions in chaos. How many miles high in the atmosphere, we couldn’t tell, but as dusk and then night came we realized we were watching the light show of a storm that was over 50 miles from our house. (Having a radar on my phone, what a miracle, showed the storm location.) This was a very tall storm, perhaps 10 miles high, and it was picking up intensity as it moved east. We watched a constant, bright barrage of lightning moving within the cloud— intra-cloud, cloud-to-cloud, and cloud-to-ground strikes. The show went on for over an hour.

Worldwide there are over 44 lightning strikes per second, nearly 1.4 billion a year. Split-second, literally 0.52 (half) second flashes are the average. Many are mere microseconds, and some bolts can last as long as 10 seconds. The current record is 17.1 seconds. This is a planetary reaction.
There are infinite sources to tell you how lightning is formed, and how to stay safe when lightning is present, I’ll leave that to the meteorologists and backcountry guides. Working in the outdoor industry for the past 35 years, lightning safety requires constant vigilance, especially when standing out on an exposed ridge while climbing one of Colorado’s 14ers. Thankfully, casualty statistics are relatively low; an average of 28 people die of lightning strikes in the US annually. Of those 28, 80% (25 of them!) are young males (ages 15-24). Hmmm.
Another obvious consequence of lightning is forest fires. As contact points that stand tall and make a direct target for the static charge to jump to, lighting can explode a tree, or tear a gash from the canopy to the ground. Up until 160 or so years ago (here’s what happened 160 years ago) fires started by lightning were generally low-level, highly beneficial fires that cleared out the forest litter and created char nutrients that feed the forest growth. I have trees on my property with obvious lightning strikes that the tree has survived and sealed the scar with resin and new bark. Other stumps left behind by logging show the char of previous fires, but the char only goes up the tree about 18 inches, a low-level burn. Forest fires now, a topic for a separate post, function on a different dynamic and burn far hotter, higher, faster, and more destructive than they used to.


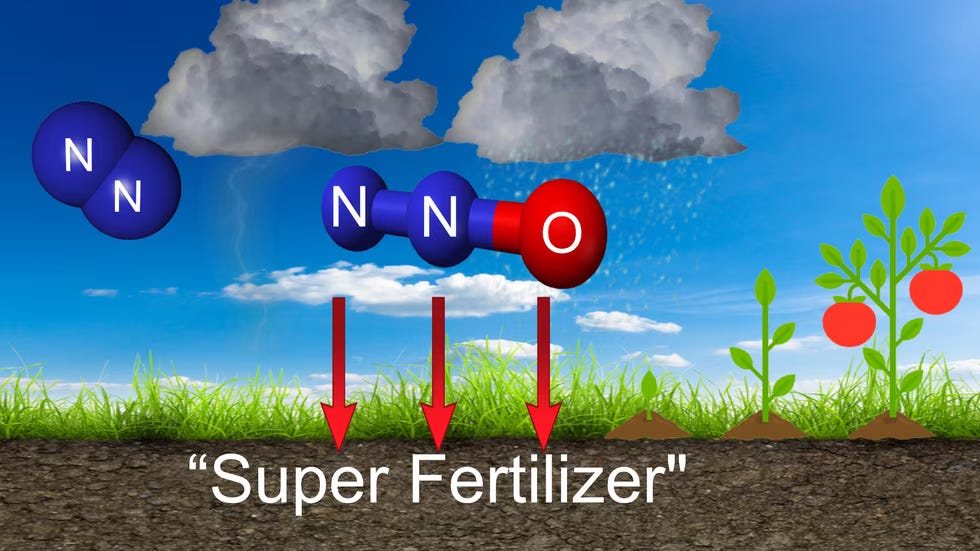
There’s even more going on with that lightning bolt than you might realize, though, so let’s dig there. Let’s dig down to the molecular level. Our air is roughly 78% nitrogen and 20% oxygen. A nitrogen molecule is two atoms bonded very tightly together, one of the strongest chemical bonds known. In this form, it is ‘non-reactive’, we breathe it in, and breathe it back out. But in this state, it cannot transfer into plants and into animal life and into us. We need nitrogen, just a little (too much and there’s trouble, but that’s another story), but it’s essential for all life. In plant life it is a super fertilizer, in humans, it is a part of cell growth, digestion, synthesizing proteins, hormones, brain, and other functions. We need a high energy, strong reaction to break apart that atomic bond to get these benefits. Here’s where lightning comes in.
Lightning splits the air, literally. Heating the surrounding air to 50,000°F (5x hotter than the sun), this heat energy splits the nitrogen atoms apart, and the single atoms begin to combine with oxygen, forming nitrogen oxide, which dissolves in water forming nitrates, and with rainfall taking it down to the ground, it can now be absorbed by plants and hence the animals and humans that eat the plants and eat the animals.
The other product of lightning is ozone. With the air split, a reactive molecule of three oxygen atoms also forms to make ozone, O3. At the stratospheric level of our atmosphere, ozone forms naturally with UV light and oxygen, O2. It forms a barrier reducing the amount of UV radiation from the sun reaching the earth’s surface, another critical role in survival on Earth. When a lightning bolt strikes cloud-to-ground, however, we can actually smell the resulting ozone in the ground-level (tropospheric) air. Some people describe it as sweet, or chlorine, or a burning odor, but you’ve smelled it in a storm. Like too much nitrogen, too much ground-level ozone becomes harmful. With lightning, it dissipates in a couple of hours.
In our scientific era of satellites and highly effective equipment to measure any number of chemicals, and dozens of points of other data, we are learning more about lightning rapidly. For such a fundamental element of life on earth, we knew little about lightning due to its magnitude, power, and relative unpredictability, but now we are finding its connection to the very sources of life in the fields of historical geology, paleolightning, geoarchaeology, and fulminology. Lightning is being traced back to the very foundations of life forming, creating amino acids, carbon, and our atmosphere. Lightning is delivering essential energy and nutrients to keep life on Earth going.
I’m sure that lightning had your respect before, - maybe less so in certain demographics, looking at you, young males, - but now you can enjoy the show and know that the lightning is providing for your life here on Earth as well.
Many thanks:
- Lightning photography by Dawn Key: https://dawn-key.pixels.com/
- Original artwork by Itsthemoon art: https://linktr.ee/Itsthemoon
- Science advisor: Dr. M Pratt
- Editing: GD Peck
- Other photo credits are The Abert Essays unless otherwise noted.
Join The Abert Essays on my social media sites:
Sources:
- https://en.wikipedia.org/wiki/Lightning#Fire_lightning
- https://www.actionnews5.com/2021/05/28/breakdown-why-lightning-is-good-agriculture/
- https://indianapublicmedia.org/amomentofscience/lightening-helps-fertilize-soil.php
- https://rmpbs.pbslearningmedia.org/resource/nves.sci.earth.nitrate/lightning-produces-nitrates/
- https://www.compoundchem.com/2018/07/31/thunderstorms/
- https://www.nps.gov/subjects/air/nature-ozone.htm
- https://www.ch.ic.ac.uk/rzepa/mim/environmental/html/nitrogen.htm